Importance of soil health
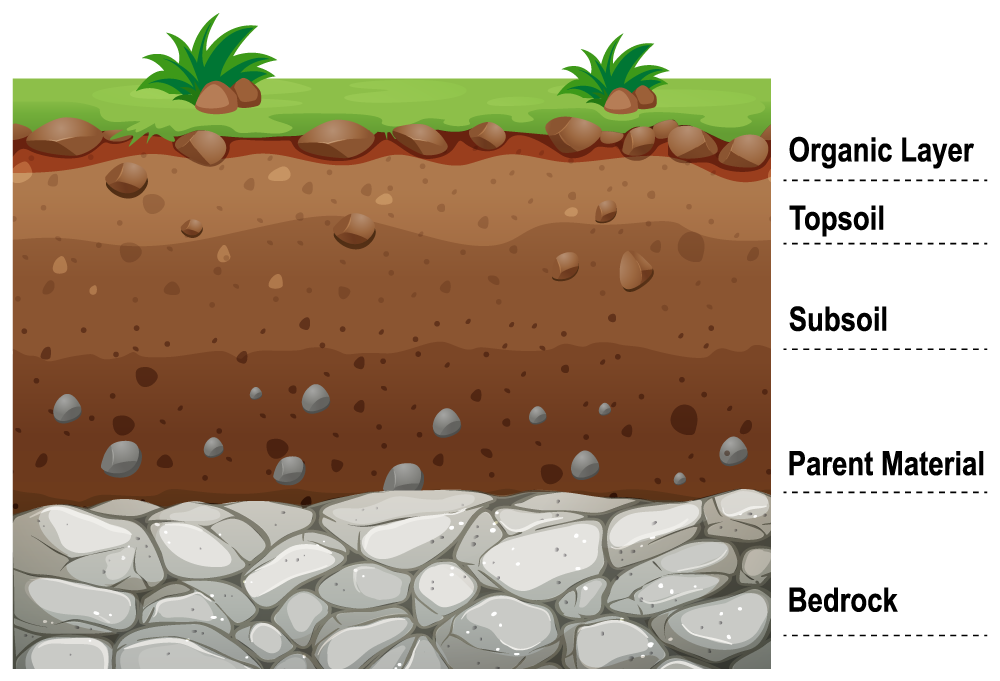
Importance of soil health
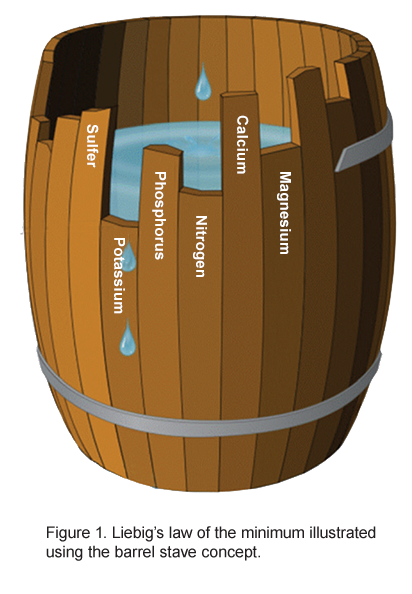
Growing healthy plants requires the proper amounts of minerals. When planting in the ground or garden beds, these nutrients will largely come from the soil. However, not all soils have the same available nutrients. Management of soil nutrients varies from place to place so the more nutrients your soil has, the better the plant will grow, right? No! There is a concept of limited nutrients called “Liebig’s law of the minimum” which outlines that if the soil nutrients are not in balance the plants will only be able to uptake enough other nutrients to be in balance with the lowest amount of another nutrient (See macronutrients below). In Figure 1, the water in the barrel represents the health and productivity of the plant. For example, if plants only have access to a certain amount of potassium they will not be able to utilize the large amounts of phosphorus or Nitrogen in the soil. The excess or lack of any one nutrient can become a limiting factor in plant growth. The following are the important nutrients that a plant requires.

Macronutrients (Required in greater amounts):
Primary– Nitrogen (N), Phosphorus (P), Potassium (K)
Secondary– Calcium (Ca), Magnesium (Mg), Sulfur (S)
Micronutrients (Required in lesser amounts): Boron (B), Zinc (Zn), Manganese (Mn), Iron (Fe), Copper (Cu), Chlorine (Cl), Molybdenum (Mb), Nickel (Ni)
Other elements that the plants need: Carbon (C), Oxygen (O), and Hydrogen (H). These elements are derived from the air (CO2) and water (H2O).
You do not supplement with these nutrients. However, more carbon stored in the soil can increase overall soil productivity (See Soil Organic Matter below)
Physical properties
Other factors, such as soil texture, also affect how the soil will support life.
Texture: The texture refers to the size of the particles in your soil. There are three categories of texture: sand, silt, and clay. Sand is the largest particle size, while clay is the smallest. These particles can be chemically identical while acting differently with water, nutrient-holding capacity, and workability.
Aggregation: When you dig a hole in the ground, is the soil you remove ever clumped together? If you answered yes, the reason is aggregation. Organic matter (carbon) from dead plants, animals, and living microorganisms hold together soil particles creating stable clumps that helps water percolate through the soil and plant roots anchor into the ground. Practices such as tilling and frequent soil disturbances break up these aggregates and can lead to less productive and unhealthy soil.
Porosity and Infiltration: Porosity refers to the space between soil particles and aggregates. Did you know that only about 45 percent of soil is minerals? Soil is made of approximately 25% water, 25% air, 5% organic material, and the rest are minerals. The water and air reside between the soil aggregates in the cracks and crevices. Having plenty of soil aggregates promotes aeration, microbial life, and water infiltration. You want water to drain into the soil when it rains rather than remaining at the surface level, causing erosion and runoff of nutrients.
Chemical properties

Cation exchange capacity (CEC)- Despite the scary-looking name, CEC is a simple concept. It is the soil’s ability to hold on to nutrients. When we apply a fertilizer or soil amendment, two things can happen to those nutrients. One option is that the nutrients are absorbed by the soil, but end up washing away next time the garden is heavily watered or during rainfall. This is not ideal because the plants did not benefit from those nutrients, money was wasted, and the nutrients can run off and harm the environment, particularly bodies of water (Ever heard of algae blooms?). What is the other outcome for those nutrients? Soil particles can bond with certain elements, holding onto them until the plants can accept them. Soils with a high cation exchange capacity (CEC) can store more nutrients than soils with a low CEC.
pH – You might have heard of pH concerning acidity and alkalinity. Generally, soil will likely be more acidic in and around forests or wetlands with pines or spruce trees. Alternatively, soils near the coast or in areas with limestone bedrock will generally be more alkaline. Different plants prefer different pH levels. Similarly to limiting nutrients, different pH levels will determine what nutrients are available to plants. The optimal range for most plants is from 6.2 to 6.7. Check out the resource below about particular plant’s preferred pH levels.
Biological properties
Soil Organic Matter (SOM): Organic matter is anything that is or has been alive in the soil. Materials like compost or manure are also organic matter. As discussed in the aggregates section, organic matter holds together soil particles. SOM can also soak up and hold onto water. This is particularly helpful during a drought and can help your plants receive water between waterings or rainfall events. As organisms decompose, they are recycled by plants and microorganisms as they break down in the soil. It’s all a big cycle feeding back into itself. The majority of organic matter in the soil will be in the top layer of the earth. This consists of decaying plants, fallen leaves, insects, and animals.
Macro and microorganisms: Another component of organic matter is the live organisms. Soil is full of life; fungi, bacteria, nematodes, insects, earthworms, etc. A healthy soil will be full of microbial life, indicating that the soil will likely be suitable for plants as well.
Interested in learning more about soils? Contact our Agriculture team at info@askaggie.org or through the submission form at the bottom of the Askaggie.org home page!
Resources about soil health
Soil Pie Chart
Liebig’s Law
Soil Aggregation
Soil pH
Cation Exchange Capacity
Cornell Soil Health Video
Written by Garrett Richards, agAlaska Soil Tech


Comments are closed